When the Patient Protection and Affordable Care Act began to transform American healthcare in 2010 Jason Haider saw an opportunity to build a company dedicated to solving two pressing challenges of the day: meeting the law’s call for measurable outcomes and lowering the cost of care while also tackling the menace of antibiotic-resistant surgical infections.
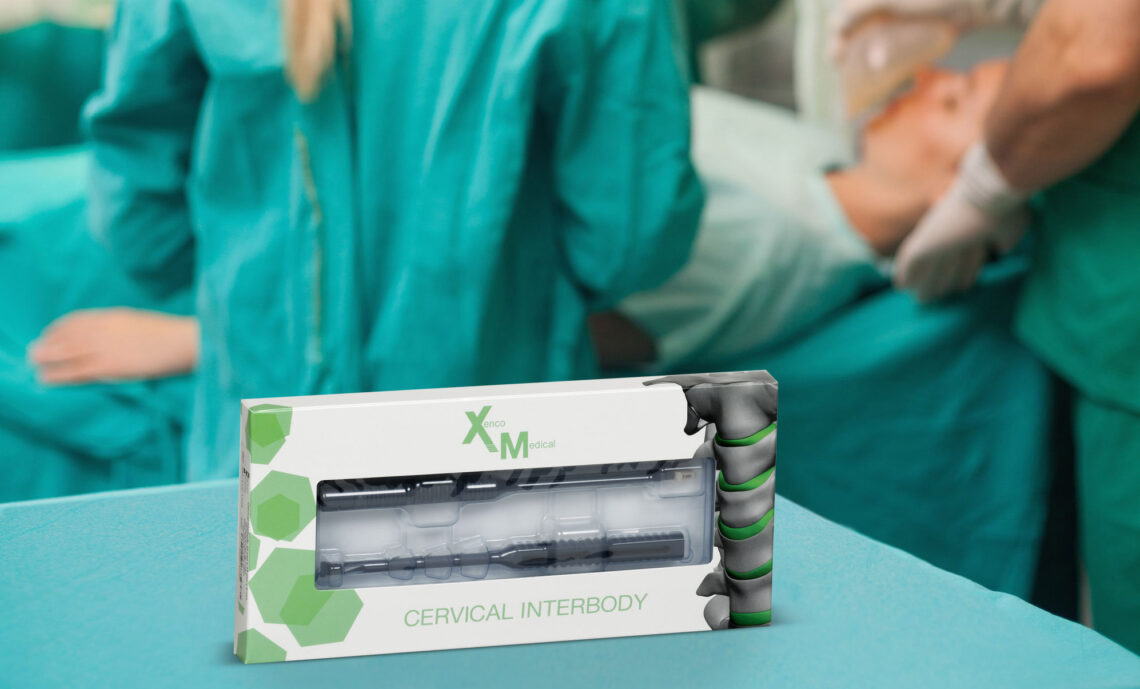
To that end Xenco Medical, the company Haider founded in 2011, pioneered a new category of surgical implant systems with sterile-packaged spinal implants preattached to disposable, composite polymer delivery instruments. Developed at the intersection of materials science and mechanical engineering, the highly reinforced, composite polymer instruments included with each of Xenco Medical’s implants are designed for both consistent mechanical performance and the elimination of the costly logistics associated with the sterilization process. They also help curb infections associated with re-using the same surgical instruments on multiple patients.
The invention of the disposable composite polymer model disrupted legacy metal-based spinal device and instrument manufacturers such as Stryker, Zimmer Biome and Medtronic.
But Xenco Medical didn’t stop there. It additionally disrupted delivery, setting up WiFi-enabled vending machines in hospitals for real-time inventory monitoring of its surgical implant systems. It has further transformed operating rooms by introducing the world’s first glasses-free holographic surgical simulation platform, enabling surgeons to translate two-dimensional datasets such as CT and MRI scans into interactive, holographic reconstructions on a light field display.
The San Diego, California-based company’s technology is now in use in 612 healthcare facilities across the United States and Latin America. To get where it is today the company, the winner of The World Economic Forum’s 2022 New Champions Award for Excellence in Sustainable Growth, had to overcome ergonomic and manufacturing challenges, navigate complex regulatory and technical landscapes, and surmount the skepticism of hospital administrators and surgeons.
“Xenco Medical embodies the characteristic of companies we are looking for and developing in the World Economic Forum’s New Champions community, a group of innovative and purpose-driven mid-sized companies,” says Julia Devos, the Head of the Forum’s New Champions community,
From Autoclaves To Wi-Fi Enabled Vending Machines
Haider’s inspiration for starting Xenco came from reading of a book entitled Superbug: The Fatal Menace of MRSA. The book described graphic examples of orthopedic surgeons grappling with the implications of antibiotic-resistant bacteria infecting their patients. “With both broad acceptance about the growing challenge of antibiotic-resistant bacteria and a government-mandated push to report and improve hospital metrics, I sought a way to tackle pathogens while reducing healthcare costs,” says Haider, an engineer with a background in life sciences.
Extensive research, which included conversations with healthcare professionals, made it clear that the surgical sterilization workflow was “an antiquated process rife with inefficiencies that left patients increasing vulnerable to pathogen exposure and added significantly to the cost of care,” says Haider. Autoclaves, which were invented in 1879 and until Xenco came along were the primary way surgical instruments were sterilized, work with a combination of steam, pressure, and time, operating at high temperature and pressure to kill microorganisms and spores.
Haider quotes a study that estimates.the sterilization processing time in autoclaves for traditional, reused metal instrument systems to be 3 hours and 28 minutes between each procedure “This is an unacceptable delay in the delivery of surgical care,” says Haider. The average time for a type of neck surgery that involves removing a damaged disc to relieve spinal cord or nerve root pressure is 2.3 hours. If you add in the time for sterilization, the number of surgeries that can be performed per day is limited. “Our systems save healthcare facilities thousands of dollars per surgery both through the elimination of sterilization costs after surgery attributable to the energy intensive steam sterilization cycle and corresponding labor as well as in enabling facilities to perform additional surgeries each day, expanding their impact on their respective communities while bolstering their revenue,” says Haider.
Because the autoclave process lacks sufficient traceability and was designed around metal-based instruments, a disruptive alternative required a different sterilization method, robust traceability, and a cost-effective material to underpin the technology, he says. Leveraging expertise from materials science consultants and undertaking an exhaustive process to meet regulatory requirements, Xenco invented a fiberglass-reinforced nylon composite compatible with gamma sterilization that promises optimal performance, lower healthcare costs, and traceable sterilization.
To achieve better outcomes at lower costs, it was important to also construct a new framework for delivery, says Haider. “I wanted to develop an automated dispensing solution around our sterile systems early on but waited until we had several years’ worth of feedback from both surgeons and hospital administrators from around the country,” he says.
Haider says his concept of a surgical vending machine was born by observing different forms of automationover time, with automated vending kiosks in airports serving as an early example. Challenges included a need for secure access and traceability as well as ease of use for hospital staff, he says.
Technical And Regulatory Hurdles
In developing its composite polymer surgical technology as well as its WiFi-enabled surgical vending machines, Xenco navigated a complex technical and regulatory landscape. For the composite polymer surgical systems, the challenge was rooted in developing high performance instruments that are also cost-effective to manufacture, says Haider. “In designing each spinal system, both ergonomics and manufacturing were driving factors in choosing the fiber orientation and mold design for each instrument,” he says. “The simultaneous durability and disposability of our lightweight yet remarkably resilient composite polymer instruments is attributable to their unique interfacial bond strength, allowing for a robust interaction between a matrix phase of semi-crystalline nylon, with its high impact strength and low internal tension, and a dispersed phase of uniquely oriented fibers.” As a surgeon exerts force on the instrument during surgery, the high interfacial bonding is important in transmitting the stress from the matrix phase to the dispersed phase, which maximizes the instrument’s overall strength, he says. With the ergonomic and manufacturing challenges overcome, an extensive regulatory process was undertaken to validate the bio compatibility, functionality, and efficacy of each of Xenco’s spinal implants preattached to its composite polymer instruments. This included establishing a rigorous packaging and sterilization program that uses the radioactive isotope cobalt 60 to penetrate sealed packages and kill any potential microorganisms before being sent to the hospital.
To date Xenco has achieved 14 approvals from the U.S. Food And Drug Administration (FDA) for nine different products covering implants for the cervical, thoracic and lumbar regions of the spine. “Each region requires its own mechanical testing and approval process,” he says, and one of its products required two because it additionally involved a fixation plate.
“It is a very demanding process,” says Haider. “There isn’t any book that teaches you the extent to which the FDA process is a negotiation.” The process involves interfacing with a reviewer in the federal government. “You have to walk them through what the technology is intended to do and why it should be approved,” he says.
For its WiFi-enabled vending machines, the challenge was rooted in designing an optimal user experience, seamless dispensing, and meeting a hospital’s cybersecurity standards, says Haider. Equipped with Wi-Fi and a touchscreen interface, each of its surgical vending machines employs an advanced elevator-based system that allows hospital staff to retrieve individual sterile-packaged systems. Each machine can hold up to 260 Xenco Medical boxes and they can be customized to meet the needs of a healthcare facility. Because they are digitally connected both the hospital and Xenco are alerted when refills are needed.
Overcoming Skepticism
In order to bring its surgical devices into hospitals and establish Xenco as partners, the company allowed hospitals to trial its technologies for free for an average of eight weeks. This enabled both the surgeons and healthcare facilities to accumulate data on the efficacy of Xenco’s approach and to realize measurable time and cost savings over a period of two months, says Haider. Individual surgeons were able to test the products both in dry labs and in cadaver labs in a simulated surgical setting, “giving them a sense for the efficiencies gained and the intuitive nature of our products,” he says.
Xenco gained its first customer in 2014 after several meetings with both the chair of the orthopedics department and members of the orthopedic service at UCLA Orthopedic Hospital in California. “It was during this process that our trial offer began to take form, and they agreed to allow the spine surgeon who was interested in our technology to use our implant and instrument system for several cases over a period of several weeks without reimbursement,” says Haider. “Once the surgeon and operating room staff had submitted their evaluation to the department, we were accepted into the hospital’s approved vendor list.”
Managing Growth
Xenco achieved profitability early on through strategic licensing agreements that garnered royalty payments and the scale of distribution it achieved through its sterile-packaged model. The company has not raised outside capital.
In 2017 Xenco tripled its staff after its fourth FDA clearance to ensure it had the operational support, regulatory oversight, and quality assurance to meet the demand of widespread distribution. “As we’ve scaled over the years, we’ve built an extraordinary team of some the most gifted engineers across our mechanical, biologics, and software departments, and we’ve maintained our company culture by equating leadership with service,” says Haider. “Every member of our team, across all specialties, is empowered as a leader and encouraged to enact their leadership through acts of service to colleagues and suppliers.”
Xenco currently operates in three countries and expects to expand into two others by the end of this year.
Lessons Learned
For Xenco succeeding meant meeting requirements in mechanical testing, bio compatibility, document control, sterilization validation, supply chain traceability and input/output verification validation.
“If you want to innovate in the medical device sector you need to respect and embrace the extent that every idea has to run its course through this gamut and understand that there is a long journey from conceptualization to commercialization,” says Haider, “While things move at a glacial speed the impact is so incredibly high and is so rewarding it makes that journey worth it.”
This article is content that would normally only be available to subscribers. Sign up for a four-week free trial to see what you have been missing.
To access more of The Innovator’s Future-Ready SME articles click here.